How is a lithium-ion battery built?
The cell of a lithium-ion battery is actually a stacked bundle of aluminium and copper plates separated by a plastic separator film (jelly roll), which some manufacturers place in a plastic pouch, others in an aluminium casing. The pouch is filled with electrolyte and sealed, and the cell is basically ready to be used to build modules, and then a battery pack from the modules.
First the electrodes have to be prepared. In the case of a cathode, the binder and solvent are added to a pre-mixed powder mixture (nickel-manganese-cobalt powder containing lithium, so-called NMC powder) in the right proportions, and the resulting slurry is then spread on aluminium foil coils in a very precise micrometre thickness in a high-purity environment.
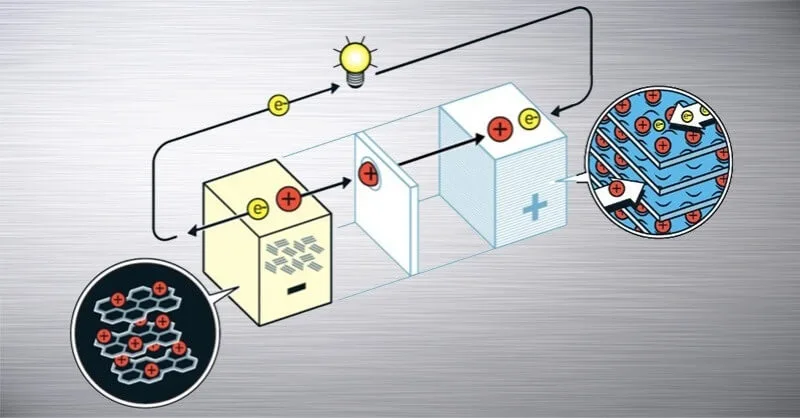
A similar process is used for the anode. Here carbon powder is used and the binder used is dissolved in water instead of solvent. The anode sludge is then coated on copper foil in a similar small thickness.
Then, in most cases, the anode and cathode are wound side by side and an insulating plastic film is placed between them to create the "jelly roll". This is a very efficient solution, but recycling is more difficult, as more different materials need to be separated in a smaller area to achieve an efficient recovery rate. However, for the overall waste stream, recycling rates of up to 90-95% can be achieved using efficient technology.
In the subsequent steps of the production process, the cells in plastic and/or aluminium cases are assembled into modules. A module typically contains 6 to 16 cells. The modules are clamped together, also in aluminium sockets. The modules are then assembled into packs, but this step is often carried out by car manufacturers in order to retain as much of the know-how as possible.